I have found that more etchers are switching to using my basic copper sulfate mordant for aluminium and zinc; while a few might have discovered that copper can also be effectively etched with a modified version of the original formula. One of the most important features of these copper sulfate based mordant is the possibility of regenerating the bath with the introduction of oxygen in some way. That seems to be overlooked by most and only a few serious etchers have been experimenting with these safer methods.


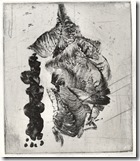
These two prints are done on aluminium by Calvin Burns in one of my classes. They are quite large and he used a larger tray
I have published my research into regeneration along with the general use of cpper sulfate on my blogsite at: http://www.ndiprintmaking.ca/?paged=3. There are other articles about using copper sulfate on previous sections of the blog and also there is information on my university server at: http://homepage.usask.ca/~nis715/. Find Intaglio mordant paper on left index column ; the university has secured a new server and for some reason some images for it don’t show up even though they seem to be sitting in my website folder – but you will not be missing much information.
Copper sulfate can be purchased in large quantities to make if less expensive. The copper floats to the surface by the hydrogen in the lines.
I discovered the use of copper sulfate in 1991-2 and had an article published in the refereed journal for the arts LEONARDO (Vol 31, No2, pp, 133-138, 1998), after it was first accepted in 1994 but then rejected because of fear the copper compounds would get into the environment. Assuring the editors that none should get into the sewer system if the etcher followed my advice, it was finally published after a second set of reviewers went over the article.
Aluminium etched with copper sulfate, salt and sodium bisulfate. The metal produces as rough surface and need not be aquatinted
Since etching is not my chosen print medium, I did the original research for the sake of saving money for my students by using aluminium instead of more expensive zinc. My research originated from my lithographic attempts of copper plating plates with cupric chloride, the experience of mixing copper sulfate with hydrochloric acid in place of the expensive cupric chloride that I didn’t have, produced corrosion instead of copper plating. This knowledge was recalled when a student asked if a cheaper metal than zinc was possible to use. In time I found that a strong enough hydrochloric acid can be made from salt and sodium bisulfate that made the copper sulfate into a very effective and safer method of etching both aluminium and zinc.
Students printing from aluminium. Yoko Imabayashi has produced the last two images. Many etchers have taken to using this soft metal
By leaving a tray of cuprous chloride open overnight after doing some research late one evening, I was surprised to fine the light green bath had dissolved all the copper particles that come from etching aluminium, to have a good green bath and no particles. It was obvious to me that oxygen played a part in regenerating the mordant and I then understood why the large plastic containers left at the printmaking department to be taken away for safe disposal, had turned dark green with various amounts of white precipitate at the bottom. As a photographer, I had discovered that developer stored in plastic allows oxygen to permeate the polyethylene and spoil the solution. I found that as long as there was some color left in the bath, it would accept oxygen to regenerate the solution by the amount of oxygen presented.
With enough salt, the mordant turns green. Air permeates the plastic containers to regenerate these bottles slated for disposal.
Ferric chloride cannot be regenerated and one has to pay for more of it and also the disposal charges.
As my teaching at the university showed up the small problems with this new safer mordant, I was able to refine the process over the many years, mostly for the sake of etchers and to be able to etch copper and not the more dangerous ferric chloride. Regeneration of the bath not only saves money but is better for the environment and the planets’ future.
I preferred to use toner images on which I rolled thin shellac that has be colored with dye. Turpentine removes the toner and does not touch shellac
I have noticed that some etchers found regenerating an interesting and important feature, but had not found my articles and research. They somehow had found the regeneration suggestions used by the circuit board manufacturers of electronic equipment. There one finds some companies using chlorine as one of most effective methods, but restricted to stiff control on using this dangerous chemical. This is not necessary for the average etching facilities in studios and teaching institutions. I had found an Australian electronic hobbyist who had a wonderful website explaining how scrap copper, hydrochloric acid and hydrogen peroxide could start a copper etching solution that could be regenerated with the addition of peroxide and sometime more acid. The solution became more potent over etching more copper circuit boards. Unfortunately, this site is now restricted for some reason, but it contained much valuable information on the chemical reactions.
A mix of HCl and peroxide forms cupric chloride that will etch copper. I chose to make HCl from salt and sodium bisulfate
Since my original formula for the copper sulfate mordant contained common salt and sodium bisulfate required to keep aluminium hydroxide from forming, I only had to add some oxygen containing material to etch copper plates and regenerate the bath. This I started to research in 2007 and finally published something in 2008 on my Internet sites and Printmaking Today (Vol.18, #2, summer 2009). It was followed up in the next publication of Printmaking Today, an article on it use by Alfons Bytautas, with whom I had worked on his reaction to this surprising discovery.
Bisulfate is in many products but the best supplier is swimming pool and hot tub shops. Sani-flush it no longer available.
Slowly my discovery has been increasing in use by the more progressive etchers, even some burin engravers have started to use and promote this safer method. Fabiola Mercandetti, an engraver in Rome has found my site and we have been corresponding for many months now about her efforts to make Italian etchers more aware about safer mordents. I really appreciate her efforts to make my methods as being the source for renamed processes that originate with my earlier publication as far back as 1992.
Heating the toner image over camp stove; the above pictures of this plate being processed. More pictures of removing toner with turpentine
For some reason my efforts have been completely overlooked by most etchers and they either don’t use my discoveries or refer to others who have renamed my original research. I did not realize that Goya might have used copper sulfate alone to etch his zinc plates. Fabiola has been doing wonderful research into what materials were used by early etchers for their plates, revealing many common chemicals were being used by some etchers instead of nitric acid, which might have been hard to get. While these household materials would etch most metals somewhat slower than my process, they certainly would be safe to use on the common plate metals. She holds workshops on safer etching and use of a burin as well in many Italian cities – which I wholly support.
Solids can be filtered out and the liquid can be poured down the drain. Copper particles - aluminium hydroxide and zinc silicate are white
On my blogsite, in previous articles, you will find how to remove all toxic materials if you decide to no longer etch plates. I explain how to remove the copper, aluminium and zinc compounds by precipitating the metals and then filtering them out. The liquid can be put down the drain as metals have all been removed. In my original articles, I had stated that bisulfate was not needed if zinc was the metal being used, but I have found that the bath cannot be regenerated as bisulfate is required for all metals to keep in acidic in nature.
Metasilicate or sodium silicate are used to precipitate the metals from mordant that is to be disposed by the etcher
I must also add that table salt is not the best salt to use because it contains calcium silicate to keep it from clumping in damp weather. This forms calcium sulfate, which is insoluble Plaster of Paris used for making molds etc. The sodium bisulfate is best bought from a shop that caters to swimming pools and hot tubs. It is a mild sulfurous acid (H2SO3) used in many other crafts where a less dangerous acid is required. I chose it because salt and the bisulfate are both dry chemicals and only need to be mixed in water as needed; keeping dangerous liquid acid off the studio shelves. To precipitate zinc, one should use sodium metasilicate or plain liquid sodium silicate, also known as Waterglass. It is available from pottery suppliers, but you must know that it dries to be waterproof if spilled. The metasilicate comes as a powder and is always dissolves it water, so the best to use. I used to get it as s TSP substitute, but it no longer seems to be available in the large hardware chain in North America.
Regenerating mordant that etched copper plate. Tule is used instead of tarlatan as it can be washed if waterbased in mixed with etching is used.
One can make their own waterbased in from Lascaux screen base, a retarder and dry pigments.
In one of the recent e-mails’ from Fabiola, she told me about a stone lithographer friend of hers’ who tried using a mix of salt and sodium bisulfate instead of nitric acid for the gum etch in traditional lithography. The first attempts were not perfect, but in the end he got good results and I would like to hear more about his research. My prediction is that there forms a thin layer of calcium sulfate (Plaster of Paris) on the stone surface, which would be more hydrophilic that calcium nitrate. I wonder how far that research will go as I no longer have any stones and only print using waterless lithography.
You should go to previous entries on my blogsite as there is information on copper sulfate mordant as well as other interesting articles. They go back to 2007.































Blog Publish
Regeneration of Copper Sulfate Based Mordant | New Directions in Printmaking
ironing ullenwood
Regeneration of Copper Sulfate Based Mordant | New Directions in Printmaking